Lithium batteries are new types of high-energy batteries that were successfully developed in the 20th century. They can be understood as batteries containing lithium elements (including metal lithium, lithium alloys, lithium ions, and lithium polymers) and can be classified into lithium metal batteries (rarely produced and used). Lithium-ion batteries (used in large quantities today). Because of its high specific energy, high battery voltage, wide operating temperature range, long storage life, etc., it has been widely used in military and civilian small appliances, such as mobile phones, laptops, camcorders, cameras, etc., partially replacing traditional batteries. .
The Origin and Development of Lithium Ion Battery
In the 1970s, Exxon MSWhittingham used titanium sulfide as a positive electrode material and lithium metal as a negative electrode material to make the first lithium battery.
In 1980, J. Goodenough discovered that lithium cobalt oxide can be used as a cathode material for lithium-ion batteries.
RRAgarwal and JR Seelman of the Illinois Institute of Technology in 982 found that lithium ions have the property of intercalating graphite. This process is rapid and reversible. At the same time, lithium batteries made of lithium metal have attracted much attention for their safety concerns, so people have tried to make rechargeable batteries using the characteristics of lithium ion-implanted graphite. The first available lithium-ion graphite electrode was successfully tested by Bell Laboratories.
In 1983 M. Thackeray and J. Goodenough et al. found that manganese spinel is an excellent cathode material with low price, stability, and excellent conductive and lithium conducting properties. The decomposition temperature is high, and the oxidizing property is far lower than that of lithium cobalt oxide. Even if a short circuit or overcharge occurs, the danger of burning or explosion can be avoided.
In 1989, A. Manthiram and J. Goodenough discovered that using a positive electrode of a polymeric anion would produce a higher voltage.
In 1991 Sony released the first commercial lithium-ion battery. Subsequently, lithium-ion batteries have revolutionized the face of consumer electronics.
In 1996, Padhi and Goodenough discovered that phosphates with olivine structure, such as lithium iron phosphate (LiFePO4), are more superior than traditional cathode materials, and thus have become the current mainstream cathode materials.

Li-ion batteries (Li-ion Batteries) are developed from lithium batteries. So before introducing Li-ion, introduce the lithium battery first. For example, button batteries are lithium batteries. The positive electrode material of a lithium battery is manganese dioxide or thionyl chloride, and the negative electrode is lithium. After the battery is assembled, the battery has voltage and does not need to be charged. This kind of battery can also be charged, but the cycle performance is not good, in the charge-discharge cycle, lithium dendrites are easily formed, resulting in internal short circuit of the battery, so under normal circumstances this kind of battery is forbidden to charge.
Later, Sony of Japan invented a lithium battery using a carbon material as a negative electrode and a lithium-containing compound as a positive electrode. In the charge-discharge process, no lithium metal exists and only lithium ions exist. This is a lithium-ion battery.
In the early 1990s, Japan's Sony Energy Development Corporation and Canada's Moli Energy Co., Ltd. respectively developed a new type of lithium-ion battery that not only performs well but also has no pollution to the environment. With the rapid development of information technology, hand-held machinery and electric vehicles, the demand for high-efficiency power supplies has increased dramatically. Lithium batteries have become one of the most rapidly developing areas.
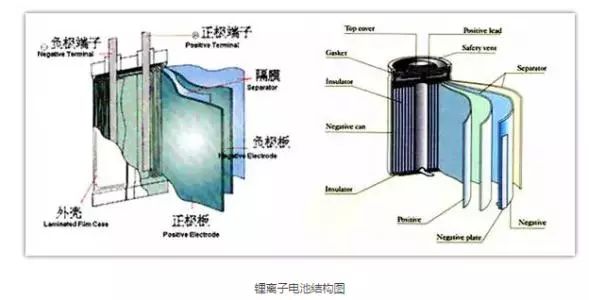
Lithium-ion battery structure and principle
The main components of lithium ion battery:
(1) Positive Electrode: The active material mainly refers to lithium cobaltate, lithium manganate, lithium iron phosphate, lithium nickelate, lithium nickel-cobalt-manganese oxide, and the like. The conductive current collector generally uses an aluminum foil having a thickness of 10 to 20 μm.
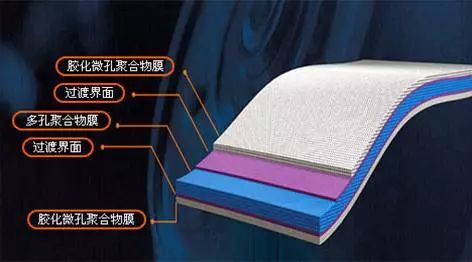
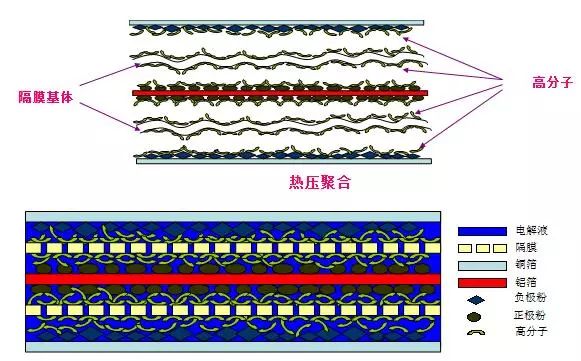
(2) Diaphragm - A special plastic film that allows lithium ions to pass through, but it is an electronic insulator. At present, there are mainly two kinds of PE and PP and their combinations. There is also a class of inorganic solid membranes, such as alumina membrane coating is an inorganic solid membrane;
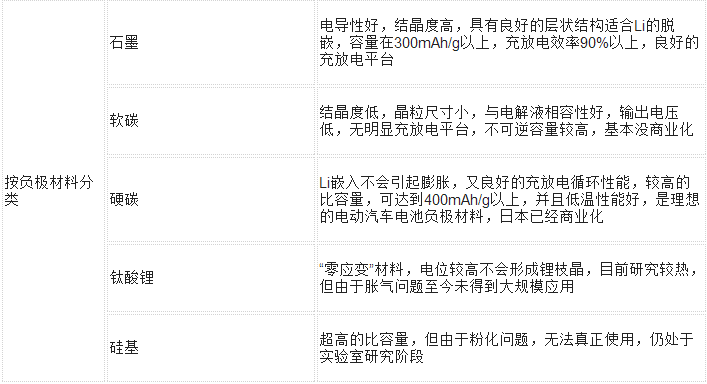
(3) Anode: The active material mainly refers to graphite, lithium titanate, or approximately graphite-structured carbon materials, and conductive collectors generally use copper foil with a thickness of 7-15 microns.
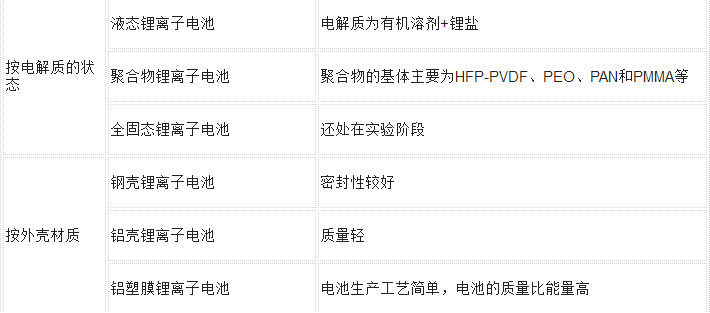
(4) Electrolyte - generally organic system, such as carbonate solvent dissolved lithium hexafluorophosphate, and some polymer batteries use gel electrolyte;
(5) Battery Enclosures - These are mainly classified into hard shells (steel shells, aluminum shells, nickel-plated iron shells, etc.) and soft bags (aluminum-plastic film).
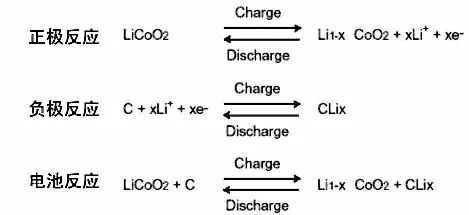
When the battery is charged, lithium ions are deintercalated from the positive electrode and embedded in the negative electrode, and vice versa. This requires that an electrode is in a lithium intercalation state prior to assembly, and a lithium-intercalating transition metal oxide having a potential of more than 3 V relative to lithium and stable in air is generally selected as the positive electrode, such as LiCoO2, LiNiO2, and LiMn2O4.
As a material for the negative electrode, an intercalated lithium compound having a potential as close as possible to a lithium potential is selected, for example, various carbon materials including natural graphite, synthetic graphite, carbon fiber, mesophase carbon, etc., and metal oxides, including SnO, SnO2, Sn composite oxide SnBxPyOz (x = 0.4 ~ 0.6, y = 0.6 ~ 0.4, z = (2 + 3x + 5y) / 2) and so on.
The electrolyte uses a mixed solvent system consisting of LiPF6 alkyl carbonate (EC), propylene carbonate (PC), and low-viscosity diethyl carbonate (DEC).
Diaphragm microporous polyolefin membranes such as PE, PP or their composite membrane, especially PP / PE / PP three-layer membrane not only lower melting point, but also has a high puncture strength, played a role in thermal protection.
The shell is made of steel or aluminum material, and the cover body has the function of explosion-proof and power-off.
Basic working principle
When the battery is charged, the lithium-containing compound of the positive electrode releases lithium ions, and the lithium ions move to the negative electrode through the electrolyte. The carbon material of the negative electrode has a layered structure, and it has many micropores. Lithium ions reaching the negative electrode are embedded in the micropores of the carbon layer. The more lithium ions are embedded, the higher the charging capacity.
When the battery is discharged (ie, we use the battery), the lithium ions embedded in the negative carbon layer come out and move back to the positive electrode. The more lithium ions returning to the positive electrode, the higher the discharge capacity. The battery capacity we usually refer to is the discharge capacity.
During the charge and discharge of a lithium ion battery, lithium ions are in a state of movement from a positive electrode → a negative electrode → a positive electrode. This is like a rocking chair. The two ends of the rocking chair are the two poles of the battery, and lithium ions move back and forth across the rocking chair. So lithium-ion batteries are also called rocking-chair batteries.
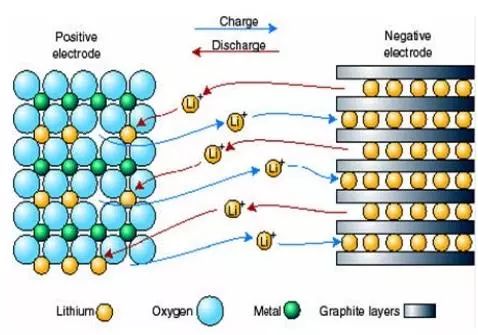
Charge and discharge mechanism
Lithium-ion battery charging process is divided into two phases: constant current charging phase and constant voltage current decreasing charging phase.
Overcharge and discharge of lithium-ion batteries will cause permanent damage to the positive and negative electrodes. Excessive discharge leads to the collapse of the negative carbon layer structure, and the collapse will cause the insertion of lithium ions in the charging process; overcharging will cause too much lithium ions to be inserted into the negative carbon structure, causing some of the lithium ions to no longer be released.
Lithium-ion batteries have the best charge/discharge method for shallow discharge. Typically 60% DOD is 2 to 4 times the cycle life at 100% DOD.
Lithium-ion battery main performance index
Battery capacity
The battery capacity is divided into rated capacity and actual capacity. The rated capacity of the battery refers to the amount of electricity that the battery should provide when it is discharged at a rate of 5h to the termination voltage at an ambient temperature of 20°C ± 5°C. It is expressed as C5. The actual capacity of the battery refers to the actual amount of electricity discharged by the battery under a certain discharge condition, which is mainly affected by the discharge rate and the temperature (so strictly speaking, the battery capacity should indicate the charge and discharge conditions).
Capacity units: mAh, Ah (1Ah=1000mAh).
Battery resistance
The internal resistance of a battery refers to the resistance that the current flows through the battery during operation. There are two parts, ohmic internal resistance and polarization internal resistance. The high internal resistance of the battery will cause the battery discharge voltage to decrease and the discharge time to shorten. The internal resistance is mainly affected by the battery's material, manufacturing process, battery structure and other factors. Battery internal resistance is an important parameter to measure battery performance.
Voltage
The open circuit voltage refers to the potential difference between the positive and negative electrodes of the battery when the battery is in a non-operating state, that is, no current flows in the circuit. Under normal circumstances, the open-circuit voltage of a lithium-ion battery is about 4.1-4.2V after full charge, and the open-circuit voltage after discharge is about 3.0V. Through the detection of the open circuit voltage of the battery, the state of charge of the battery can be judged.
Operating voltage, also known as the terminal voltage, refers to the potential difference between the positive and negative electrodes of a battery when the battery is in operation, that is, when current flows through the circuit. When the battery is discharged, when the current flows through the battery, there is no need to overcome the resistance caused by the internal resistance of the battery. Therefore, the working voltage is always lower than the open circuit voltage, and charging is the opposite. Lithium-ion battery discharge voltage is about 3.6V.
Discharge platform time
Discharge platform time refers to the discharge time to discharge to a certain voltage when the battery is fully charged. Example: A ternary battery measures its discharge platform time of 3.6V, with a constant voltage charge voltage of 4.2V, and when the charge current is less than 0.02C the charge is fully charged, and then it is left for 10 minutes at any rate of discharge current The discharge time at the time of discharge to 3.6V is the discharge plateau time at this current.
Because some working voltages of the appliances that use lithium-ion batteries have voltage requirements, if they are lower than the required values, there will be cases where they cannot work. So the discharge platform is one of the important standards to measure the battery performance.
Charge and discharge rate
The charge/discharge rate refers to the current value required for the battery to discharge its rated capacity within a specified time. 1C is numerically equal to the battery's rated capacity, usually denoted by the letter C. If the battery's nominal rated capacity is 10 Ah, then 10 A is 1 C (1 rate), 5 A is 0.5 C, 100 A is 10 C, and so on.
Self-discharge rate
The self-discharge rate, also called the charge holding capacity, refers to the battery's ability to maintain the capacity of the battery under certain conditions in an open state. Mainly by the battery manufacturing process, materials, storage conditions and other factors. It is an important parameter to measure the battery performance.
effectiveness
Charging efficiency refers to a measure of how much energy a battery consumes during charging to convert it into a chemical energy that the battery can store. Mainly by the battery process, formula and battery operating environment temperature, the higher the general ambient temperature, the charging efficiency is low.
Discharge efficiency refers to the ratio of the actual electricity discharged from the discharge to the end point voltage under a certain discharge condition to the rated capacity of the battery, which is mainly affected by factors such as discharge rate, ambient temperature, and internal resistance. Under normal circumstances, the discharge rate is higher. The lower the discharge efficiency. The lower the temperature, the lower the discharge efficiency.
Cycle life
The battery cycle life refers to the number of charge and discharge cycles that the battery experiences under a certain charge-discharge system when the battery capacity drops to a specified value. Lithium-ion battery GB regulations, under 1C conditions after the battery cycle 500 times the capacity retention rate of 60%.
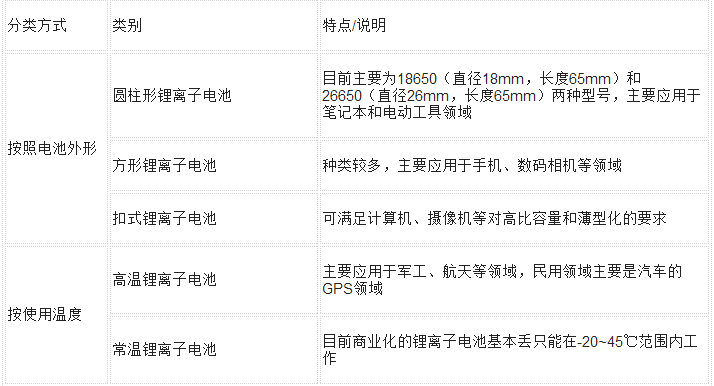
The main classification of lithium-ion batteries
(1) According to the different electrolyte materials used for the lithium battery, the lithium battery can be classified into two categories: a lithium ion battery (abbreviated as LIB) and a polymer lithium ion battery (abbreviated as LIP).
(B), according to the charging mode can be divided into: non-chargeable and rechargeable two types.
(C), lithium battery appearance points: square lithium battery (such as commonly used mobile phone batteries) and columnar (such as 18650, 18500);
(D), lithium battery outsourcing materials points: aluminum shell lithium battery, lithium battery steel shell, soft pack battery;
(5) Lithium batteries are divided into positive and negative materials (additives): lithium cobalt oxide (LiCoO2) batteries, lithium manganese oxide (LiMn2O4), lithium iron phosphate batteries, disposable manganese dioxide lithium batteries
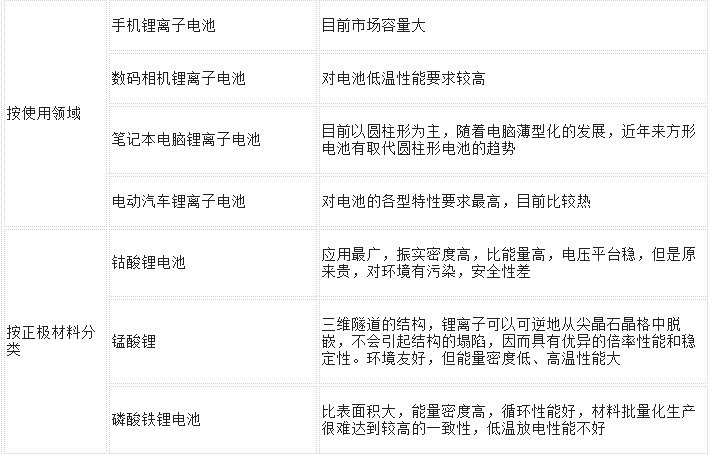
More categories are listed in the following table:
Lithium polymer battery
The positive and negative materials used in lithium polymer batteries are the same as liquid lithium. The working principle of the battery is also basically the same. Their main difference lies in the difference of electrolytes. Lithium batteries use liquid electrolytes, and polymer lithium batteries replace solid polymer electrolytes. Such polymers can be either "dry" or "colloidal." Most of the current use of polymer colloidal electrolytes.
Lithium polymer batteries can be divided into three categories:
1. Solid polymer electrolyte lithium battery. The electrolyte is a mixture of a polymer and a salt. This battery has low ionic conductivity at room temperature and is suitable for high temperature use.
2. Gel polymer electrolyte lithium battery. That is, additives such as a plasticizer are added to the solid polymer electrolyte to increase the ionic conductivity so that the battery can be used at room temperature.
3, lithium polymer battery cathode material. The use of conductive polymers as cathode materials, which is 3 times the energy of existing lithium batteries, is the latest generation of lithium batteries. Since the solid electrolyte replaces the liquid electrolyte, compared with the liquid lithium battery, the polymer lithium battery has advantages such as thinning, arbitrary area, and arbitrary shape, and does not cause safety problems such as liquid leakage and combustion explosion. Therefore, you can use aluminum-plastic composite film manufacturing battery casing, which can increase the capacity of the entire battery; polymer lithium battery can also use polymer as cathode material, and its mass ratio energy will be more than 50% higher than the current liquid lithium battery. In addition, the polymer lithium battery has an increase in operating voltage, charge and discharge cycle life, etc. than lithium batteries.
Lithium polymer battery advantages:
1, good safety performance
Lithium polymer battery in the structure of aluminum plastic flexible packaging, different from the liquid metal core of the battery, in the event of a security risk, the liquid battery is prone to explosion, and the polymer battery will only gas drum.
2, the thickness is small, can be made thinner
Ordinary liquid lithium battery using the first custom shell, after the plug positive and negative cathode material method, the thickness of 3.6mm below the technical bottlenecks, polymer batteries do not exist this problem, the thickness can be done below 1mm, in line with the current needs of mobile phones direction.
3, light weight
The weight of the polymer battery is 40% lighter than the steel lithium battery of the same capacity specification and 20% lighter than the aluminum shell battery.
4, large capacity
Polymer batteries are 10 to 15% higher in capacity than steel shell batteries of the same size and 5 to 10% higher than aluminum shell batteries, making them the first choice for color-screen mobile phones and MMS mobile phones. Nowadays, new color-screen and MMS mobile phones are also widely used in the market. Polymer batteries.
5, small internal resistance
The internal resistance of polymer batteries is smaller than that of liquid batteries. At present, the internal resistance of domestic polymer batteries can even be less than 35mΩ, which greatly reduces the self-consumption of batteries and extends the standby time of mobile phones. With the international standards. This type of polymer lithium battery, which supports large discharge currents, is an ideal choice for remote control models and has become the most promising alternative to nickel-metal hydride batteries.
6, the shape can be customized
The polymer battery can increase or decrease the thickness of the battery core according to the customer's demand, develop a new battery model, the price is cheap, the mold opening period is short, and some can even be tailored according to the shape of the mobile phone, so as to fully utilize the battery housing space and enhance the battery capacity.
7, good discharge characteristics
The polymer battery uses a colloidal electrolyte. Compared to the liquid electrolyte, the colloidal electrolyte has a stable discharge characteristic and a higher discharge platform.
8, the protection board design is simple
Due to the use of polymer materials, the battery core does not ignite or explode, and the battery itself has sufficient safety. Therefore, the design of the protection circuit of the polymer battery may consider omitting the PTC and the fuse, thereby saving the battery cost. Polymer lithium batteries have great advantages in terms of safety, volume, weight, capacity, and discharge performance.
Lithium iron phosphate battery
Lithium batteries from positive and negative materials are also divided into lithium cobalt oxide (LiCoO2) batteries, lithium manganese oxide (LiMn2O4), and lithium iron phosphate batteries.
In the first lithium battery introduced by Sony, the positive electrode material is lithium cobalt oxide and the negative electrode material is carbon. Among them, the positive electrode material mainly determines the maximum chargeable capacity and open circuit voltage of the battery.
A lithium iron phosphate battery refers to a lithium battery using lithium iron phosphate as a positive electrode material. Lithium batteries have a variety of cathode materials, including lithium cobalt oxide, lithium manganese oxide, lithium nickelate, ternary materials, and lithium iron phosphate. Among them, lithium cobalt oxide is a cathode material used in most lithium batteries, and other cathode materials have not yet been mass-produced on the market for various reasons. Lithium iron phosphate is also one of lithium batteries. From the material's principle, lithium iron phosphate is also an intercalation/deintercalation process. This principle is the same as lithium cobalt oxide and lithium manganate. Lithium iron phosphate batteries are used to make lithium secondary batteries. Now the main direction is power batteries. Compared with NI-MH and Ni-Cd batteries, there are great advantages.

Characteristics of lithium iron phosphate battery
1, long life
The long-life lead-acid battery has a cycle life of about 300 cycles and a maximum of 500 cycles, while the lithium iron phosphate power battery has a cycle life of more than 2,000 cycles, and the standard charge (5-hour rate) can be used up to 2,000 times. The lead-acid battery of the same quality is “new half year, old half year, maintenance and maintenance for another half yearâ€, and at most 1 to 1.5 years, and the use of lithium iron phosphate battery under the same conditions will reach 7-8 years. Considering comprehensively, the cost performance ratio will be more than 4 times that of lead-acid batteries.
2, the use of security
Lithium iron phosphate completely solves the potential safety hazards of lithium cobalt oxide and lithium manganese oxide. Lithium cobaltate and lithium manganese oxide will cause explosion in a strong collision and pose a threat to the lives of consumers, while lithium iron phosphate has undergone stringent Safety tests do not produce explosions even in the worst traffic accidents.
Can be high current 2C fast charge and discharge, in a dedicated charger, 1.5C charge within 40 minutes to make the battery full, starting current up to 2C, and lead-acid batteries now do not have this performance.
3, high temperature
Lithium iron phosphate electric peak up to 350 °C -500 °C and lithium manganate and lithium cobalt oxide is only about 200 °C. Operating temperature range is wide (-20C--+75C), with high temperature resistance characteristics of lithium iron phosphate electric peak up to 350 °C -500 °C and lithium manganese oxide and lithium cobalt oxide is only about 200 °C.
4, capacity
Has a larger capacity than ordinary batteries (lead acid, etc.). Rechargeable batteries are often operated under full-load conditions, and the capacity is quickly below the rated capacity. This phenomenon is called the memory effect. Like nickel-metal hydride, nickel-cadmium batteries exist memory, and lithium iron phosphate battery does not have this phenomenon, the battery no matter what state, can be used with the charge, do not need to finish before recharging.
The volume of the lithium iron phosphate battery of the same specification capacity is 2/3 of the volume of the lead-acid battery is 1/3 of that of the lead-acid battery. The battery does not contain any heavy metals and rare metals (nickel metal hydride batteries require rare metals), non-toxic (SGS certification passed), non-polluting, in line with European RoHS regulations, is an absolute green battery certificate.
5, no memory effect
Lithium-ion battery performance mainly depends on the positive and negative electrode materials. Lithium iron phosphate is a lithium battery material that only appeared in recent years. The domestic development of large-capacity lithium iron phosphate battery was in July 2005. Its safety performance and cycle life are incomparable with other materials, and these are also the most important technical indicators of power batteries. 1C cycle life up to 2000 times. Single battery overcharge voltage 30V does not burn, puncture does not explode. Lithium iron phosphate cathode materials make large-capacity lithium batteries easier to use in series. To meet the need for frequent charging and discharging of electric vehicles. With the advantages of non-toxicity, no pollution, good safety performance, wide source of raw materials, low price, long life, etc., it is the ideal cathode material for new generation lithium batteries.
The positive electrode of lithium battery is lithium iron phosphate material. This new material is not a conventional lithium battery cathode material LiCoO2; LiMn2O4; LiNiMO2. Its safety performance and cycle life are incomparable with other materials, and these are also the most important technical indicators of power batteries. 1C cycle life up to 2000 times. Single battery overcharge voltage 30V does not burn and does not explode. Puncture does not explode. Lithium iron phosphate cathode materials make large-capacity lithium batteries easier to use in series.
Lithium iron phosphate batteries also have their disadvantages: for example, the tap density of lithium iron phosphate cathode materials is small, and the volume of equal-capacity lithium iron phosphate batteries is larger than that of lithium batteries such as lithium cobalt oxide, and thus has no advantage in miniature batteries.

In the post-industrial era, the speed at which automobiles were popular surpassed our imagination. While bringing efficiency and convenience, emissions from a large amount of exhaust gas also put a lot of pressure on the environment. With the soaring price of oil and carbon dioxide emissions, greenhouses are brought into the greenhouse. The highlight of issues such as effects is the urgent need to find new energy alternatives to traditional energy sources. Liquid hydrogen, fuel cells, etc. are good choices, but there are problems such as high prices and immature technologies. The cost of ordinary lead-acid batteries is relatively low, but their weight is heavy, their energy density is low, their service life is short, and there are potential heavy metals. Pollution and other issues.

A new generation of electric vehicles using the new lithium iron phosphate battery as its power core, this green power has many characteristics and advantages:
1, very high security
To be a vehicle driver, safety is an overriding priority. Although the basic lithium battery safety can get basic guarantees, there is the possibility of fire or explosion under extreme conditions. Lithium iron phosphate battery as the second generation of lithium batteries, its physical properties are stable, and then with the built-in battery pack over-voltage, undervoltage, over-current, overcharge and other protection features, no explosion can not fire, is currently the only absolute safety in the world Lithium Ion Battery. Due to the use of high thermal stability materials and careful process design, battery safety and reliability are greatly enhanced. The lithium iron phosphate battery will not explode even if it is thrown into a fire, compared to the explosion that may occur during improper use of the lithium battery. High temperature stability up to 400-500 °C, to ensure the inherent safety of the battery; will not be due to overcharge, over temperature, short circuit, impact and explosion or combustion. After a rigorous safety test, no explosion will occur even in the worst traffic accidents.
2, long life and low cost
As a power battery, the service life (cycle performance) is closely related to the overall cost of use. Compared with the cycle life of ordinary lithium batteries of about 500 cycles, a lithium iron phosphate battery can be charged and discharged at room temperature for 1,500 cycles, and the capacity retention rate is 95%. The above, while the 50% capacity cycle life is more than 2000 times, the battery mileage is more than 500,000 kilometers, can be used for about five years, is 8 times the lead-acid battery, nickel-metal hydride battery 3 times, is cobalt Lithium battery about 4 times. In addition, the manufacturing cost itself is lower than ordinary lithium batteries, which undoubtedly can greatly reduce the use and maintenance costs of electric vehicles.
At the same time, the discharge performance of lithium iron phosphate battery is also very good, the power curve is stable, and the resistance to over-discharge is strong. After ordinary lithium batteries are lower than 3.2V, discharge is overdischarge, which may lead to scrap. However, the lithium iron phosphate battery has energy release at 2.8V, and there is no problem of scrapping below 2.5V.
3, easy to use and handle
We know that nickel-metal hydride and nickel-cadmium batteries have a strong memory effect. Ordinary lithium batteries also have a certain memory effect. They need to be “full and full†as far as possible, which can cause inconvenience to the daily use of electric vehicles, and lithium iron phosphate batteries are not available. This phenomenon, small self-discharge; no memory effect, regardless of the state of the battery, can be used with the charge, do not have to finish before recharging, while the battery fast charge characteristics, with a dedicated charger fast charge, half an hour can be adequate 95 %about. The treatment problem after the end of the battery life also deserves our attention. The lithium iron phosphate battery does not contain any heavy metals and rare metals. It is non-toxic, non-polluting and meets the regulations. It is an absolute green battery, and there is a lot of lead in the lead-acid battery. , If it is handled improperly after it is discarded, it will cause secondary pollution to the environment, and the lithium iron phosphate material will not be polluted in production and use.
Bitcoin mining equipment is mainly highly specialized ASIC mill , The maximum computing power of a single miner is 110T/s( Ant S19Pro mill ), The scale of computing power of the whole network is 120EH/s above . Ethereum's mining equipment is mainly graphics card mining machine , Professional ASIC Mining machines are very few , On the one hand, it is because of the of Ethereum mining algorithm [ resist ASIC sex " Improved R & D efficiency ASIC The threshold of the miner , So how much do you know about the mainstream Ethereum mining machine ?
Ethereum mining algorithm has higher requirements for memory , The purpose of this design limits ASIC The use of chips in Ethereum mining . From the results , Most Ethereum mining machines are graphics card machines , This reflects the application of Ethereum mining algorithm ASIC Success on . From the absolute value of computational power scale , The computing power of Ethereum is about 200TH/s, The computing power of bitcoin is about 120EH/s, The latter is close to the former 60 ten thousandfold .
ASIC Mining machines have high computing power , Large power consumption , Like the latest ant S19Pro mill , Rated power consumption is 3250W, Need to consume... Every day 78 Degree electricity , According to the current currency price and 0.23 The electricity price in wet season is RMB , The proportion of electricity charge is 30.68%. Other older bitcoins ASIC mill , Like ants T17 series , The proportion of electricity charges generally exceeds 50%. by comparison , The power consumption of the graphics card miner is low , The proportion of electricity is also low . such as 5600XT8 Graphics card miner of card .
Ant S19-95T It adopts aluminum alloy profile electromechanical integrated design , Parallel four fan structure . According to the measured data , In line with and better than the official data .S19 The series is provided by TSMC 7nm chip , A new generation of customized chips with perfect whole machine architecture and high conversion rate APW12 Power Supply , Reduce power loss during conversion , Use more energy-saving , Greatly shorten the return cycle of the miner . In addition, the newly upgraded mining machine operation interface is also more humanized , Real time computing power . The average computing power and the operation of each chip are clear at a glance .
Shenma mining machine M30S-86T/88T/90T, Bit micro 2020 Launched in 2013 M30S Series of new mining machines , Announced that it would M30S The series is expanded to three 3X product . stay 3X In the new standard , Bit micro promises a one-year warranty for its series of products . This series of mining machines are provided by Samsung 8nm chip , Among them, god horse M30S series , The power consumption is 38J/T, Provide higher computing power , Lower power consumption and high stability.
Ethereum Mining Machine:Jasminer X4,Bitmain Antminer E9 (2.4Gh),iPollo V1,Jasminer X4-1U,iPollo V1 Classic,
Jasminer X4-C 1U,iPollo V1 Mini SE Plus
Ethereum Mining Machine,ETHW Miner,innosilicon a10 pro,E9 Antminer,Innosilicon A11
Shenzhen YLHM Technology Co., Ltd. , https://www.asicminer-ylhm.com
